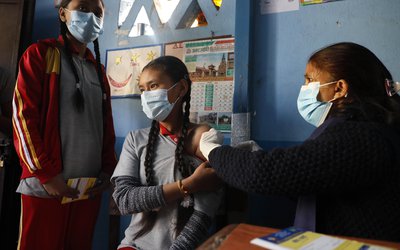
Phase 2/3 human clinical trials of the Oxford University-AstraZeneca vaccine candidate for Covid-19, manufactured by Serum Institute of India, will begin in the coming week.
Three-four trial sites have demonstrated the requisite preparedness, and doses of the candidate vaccine could be administered as early as Monday or Tuesday, top officials at the Indian Council of Medical Research (ICMR) said.
The government has allowed fast-tracking of regulatory clearances for the vaccine against the novel coronavirus; the final approval was related to certification from the Directorate General of Health Services’ Central Research Institute in Kasauli, Himachal Pradesh, for use of the investigational product in Phase 2/3 clinical trials.
The trials can begin simultaneously at all sites that are prepared, ICMR officials said. Four of the 14 trial sites are in Pune, and two are in Mumbai. The ICMR-Regional Medical Research Centre, Gorakhpur, and two other sites will not be part of the clinical trials.
The Oxford-AstraZeneca candidate – ChAdOx1 nCoV-19 (AZD1222) – has shown encouraging results in early human trials. Pune-based Serum Institute, the world’s largest manufacturer of vaccines, is conducting Phase 2/3 clinical trials of the candidate, which has been named Covishield in India.
Dr Sanjay Lalwani, Medical Director of Pune’s Bharati Vidyapeeth Deemed University Medical College and Hospital, said: “We are ready to start the trial, and are waiting for official communication from the authorities. If the vaccine doses are sent on Monday, we can begin Phase 2 clinical trials on Tuesday.”
He said several people keen to participate in the trial had called and emailed. “Many people want to enrol. We have committed to enroll 350 trial participants,” Dr Lalwani said.
Pathik Divate, CEO of Jehangir Clinical Development Centre, another trial site in Pune, also said they were waiting for the vaccines and were ready to start the trial. Approximately 250-300 participants would be enrolled, he said – the criteria for enrolment include being healthy, above 18 years of age, and not having been infected with the virus.
The other two trial sites in Pune are KEM Hospital and Research Centre, Vadu, and B J Medical College and Sassoon General Hospital.
The serum has a partnership with AstraZeneca to manufacture the ChAdOx1 nCoV-19 vaccine in India. Covishield will be released for commercial use after the trials are successfully completed, and regulatory approvals are in place, the Institute said in a release on Sunday.
This will be a double-blind (meaning neither the researchers nor the participants will know who is getting the vaccine and who is getting a placebo) randomized clinical trial. According to the Clinical Trials Registry (CTR) India, of the 1,600 subjects of the trial, 400 will be part of the immunogenicity cohort, and the remaining 1,200 will be randomly assigned in a 3:1 ratio to receive either Covishield or a placebo. Covishield will be administered as two 0.5 ml intramuscular doses on Day 1 and Day 29 of the trial.
Source: The Indian Express
- Qatar And Nepal Ink Several MoUs, Returned Home Completing Two Days Visit
- Apr 24, 2024
- Russia Industrialists Met Prime Minister Dahal
- Apr 24, 2024
- President Paudel Hosts Banquet In Honour Of Visiting Qatari Emir
- Apr 24, 2024
- Weather Forecast: Thunder Showers Is Likely At Few Places Of Koshi, Karnali And Sudur Pashchim
- Apr 24, 2024
- Japan Hands Over The Community Center For Disaster Prevention
- Apr 23, 2024