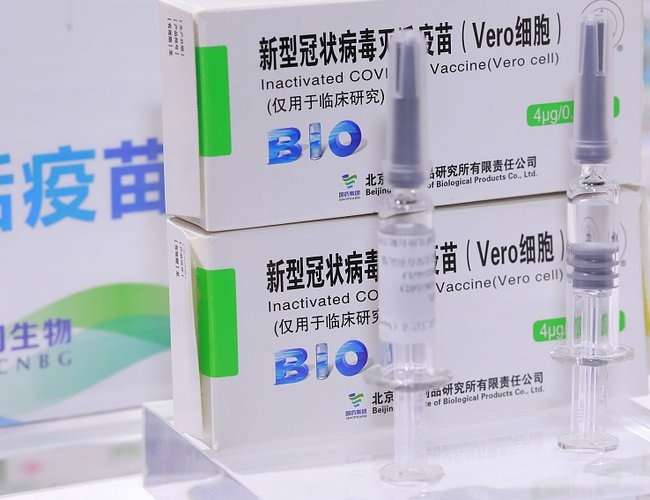
UBAI (Reuters) - Bahrain said on Sunday it had approved a COVID-19 vaccine developed by China National Pharmaceutical Group (Sinopharm) and launched online registration for the vaccine for citizens and residents.
A statement by the National Health Regulatory Authority did not specify which vaccine of two being developed by Sinopharm had been given the go-ahead, but cited data from Phase III clinical trials that showed an 86% efficacy rate and said Bahrain had participated.
Bahrain’s Ministry of Health said in a statement and on its Instagram account that citizens and residents above 18 years of age could register online to receive the vaccine for free.
The data cited was the same as announced earlier this month by the United Arab Emirates from an interim analysis of late-stage clinical trials for an inactivated vaccine developed by the Beijing Institute of Biological Product, a unit of Sinopharm’s China National Biotec Group (CNBG).
In July, the UAE started Phase III clinical trials for the vaccine and the trial was expanded to Bahrain, Jordan and Egypt.
Neither CNBG nor Sinopharm was available for comment.
- DigitALL: Innovation And technology For Gender Equality
- Mar 08, 2023
- India Says It Will Protect Its Interests As Chinese Boat Heads To Sri Lanka
- Jul 30, 2022
- Omicron Poses 'Very High' Global Risk, World Must Prepare: WHO
- Nov 29, 2021
- Resumption Of India's COVAX Vaccine Supply Delayed By Nepal
- Nov 24, 2021
- US Shipping 1.5 Million J&J Vaccines To Nepal
- Jul 10, 2021