The Drug Controller General of India (DCGI), the country’s national drug regulator, announced on Sunday (January 3) that the Central Drugs Standard Control Organisation (CDSCO) has decided to accept the recommendations of its Subject Expert Committee (SEC), and approved the Covid-19 vaccines of both Serum Institute of India and Bharat Biotech for restricted use in the country. What happens now?
Government will procure vaccines
Serum Institute of India (SII), has manufactured Covishield, the Indian variant of the AZD1222 vaccine developed by Oxford University and AstraZeneca, and already stockpiled some 80 million doses. As such, the rollout can begin fairly quickly.
The other vaccine that has got emergency use authorisation, Covaxin, manufactured by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR), could take a few days or weeks to be available.
Process of administering will start
In the United States and United Kingdom, the first shots were administered within 1-2 days of the Pfizer-BioNTech and Moderna vaccines receiving regulatory approval. In India too, the process is likely to be very fast. While no concrete timelines have been announced it, it can be reasonably expected that the mass vaccination programme will begin within a week — perhaps by the coming weekend.
The first recipients of the vaccine
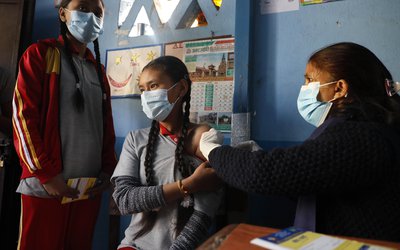
First, the entire vaccination drive will be voluntary. The government has already announced that first in line will be 30 million (3 crore) workers in the forefront of India’s battle against the novel coronavirus, including 1 crore healthcare workers and 2 crore frontline workers. Health Minister Dr Harsh Vardhan announced on Saturday (January 2) that the vaccine will be administered to them for free. Also receiving the vaccine in the first phase will be a third priority group – consisting of some 27 crore persons above age 50, and persons below age 50, but with associated comorbidities.
“In 1st phase of #COVID19Vaccination, free #vaccine shall be provided across the nation to most prioritised beneficiaries that incl[ude] 1 crore healthcare & 2 crore frontline workers. Details of how further 27 cr[ore] priority beneficiaries are to be vaccinated until July are being finalised,” Dr Harsh Vardhan tweeted on January 2.
- Students, Police Clash On US Campuses Over Gaza
- Apr 25, 2024
- Israel Procures Tents To Evacuate Rafah Residents For Ground Offensive
- Apr 25, 2024
- Nepal Gifts Two Elephants To Qatar
- Apr 24, 2024
- Israel Urges Residents In Part Of Northern Gaza To Evacuate
- Apr 24, 2024
- US Senate Takes Up Aid For Ukraine, Israel, Indo-Pacific
- Apr 24, 2024