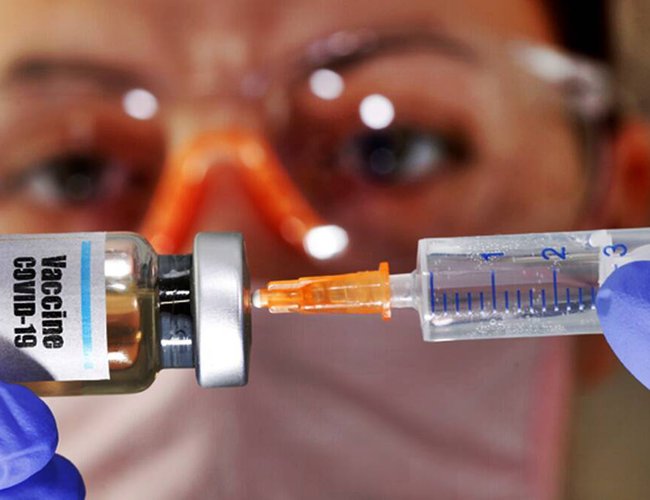
Oxford University's vaccine, being developed in collaboration with AstraZeneca, is considered the most promising to be successful, and is undergoing phase-3 trials in some other countries.
Phase-2 AND 3 trials of the Oxford University vaccine for novel Coronavirus begin in India today, with the first doses being given to a set of six volunteers at Pune’s Bharati Vidyapeeth medical college and hospital. This vaccine, developed in collaboration with AstraZeneca, is considered the most promising to be successful, and is undergoing phase-3 trials in some other countries.
Indian regulatory rules require a vaccine to be tested within India for it to be authorised for use on Indian population. Accordingly, Pune’s Serum Institute of India, which has an agreement with AstraZeneca to produce and market this vaccine in India and many other countries, has initiated human trials. Both phase-2 and phase-3 trials would take place in India. Phase-1, during which the vaccine’s safety is assessed, is not mandatory to be repeated in India.
The phase-2 trials will take place at two more locations, at the KEM hospital in Mumbai and PGI, Chandigarh. Several more hospitals would be involved in phase-3 trials, during which the vaccine’s efficacy, or its ability to protect the volunteers from the disease in real life situations, is assessed. In phase-3, the vaccine is tried on several thousand volunteers. However, since these tests are only being repeated in India, a total of 1,600 volunteers are expected to be given the vaccine during the phase-2 and phase-3 trials in India.
India says in talks with Russia on Sputnik-V vaccine
India on Tuesday said it had been discussing the possibility of bringing the Russian vaccine against novel Coronavirus, called Sputnik-V, with the Russian authorities.
“As far as Sputnik-V vaccine is concerned, India and Russia are in communication. Some initial information has been shared,” Health Secretary Rajesh Bhushan said at a press conference.
Russia has also said that India was one of the countries where the vaccine could be manufactured. It is not clear how concrete these talks have been, or whether any decision to bring the Russian vaccine to India has already been taken.
The Russian vaccine, the first to be authorised for general use, has attracted a great degree of scepticism from the global scientific community, because of the superfast speed in which it was developed and authorised, without going through phase-3 human trials, which are considered necessary to assess the effectiveness of any vaccine.
Source: The Indian Express
- Russia launches one of its largest attacks against Ukraine
- Jul 06, 2025
- India notifies WTO of proposed retaliatory duties against US tariffs on autos
- Jul 06, 2025
- PM Oli Returns Home Attending UN FFD4 Summit
- Jul 05, 2025
- Trump signs into law domestic policy bill
- Jul 05, 2025
- Putin-Trump call after US halts some arms shipments to Ukraine
- Jul 04, 2025














